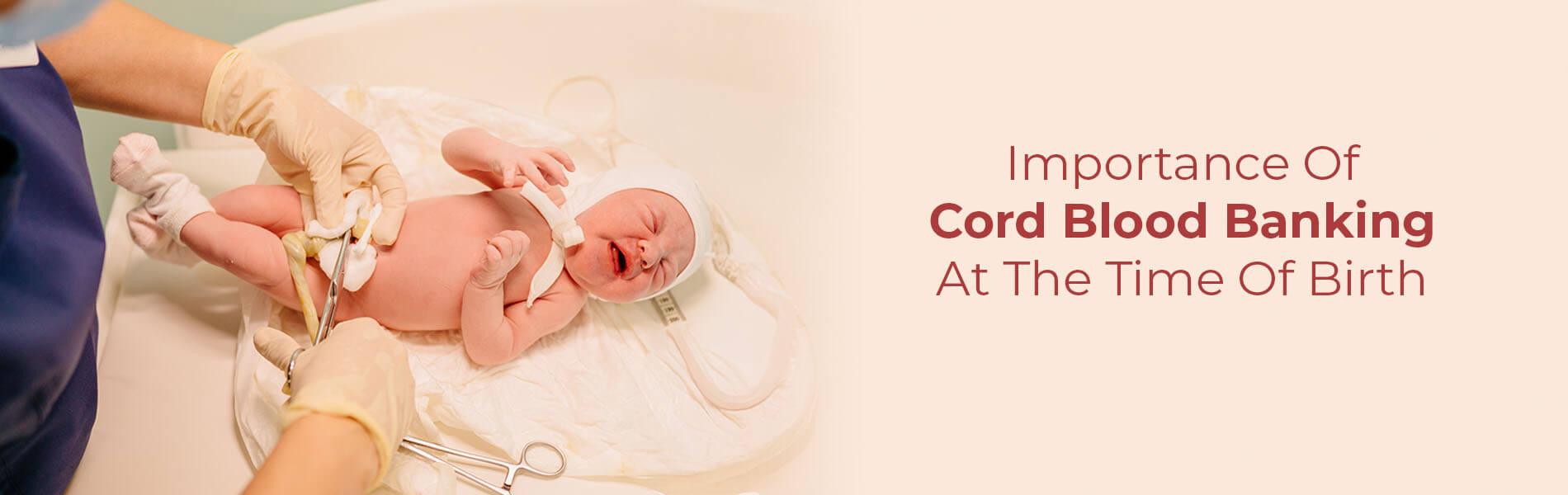
Guidelines for Umbilical Cord Blood Banking: Collection, Processing, Testing, Storage, and Release
All the new parents and parents-to-be out there ought to have heard of stem cell banking. But, is it really necessary to bank your baby’s cord blood at birth? Are these cells really capable of treating an array of genetic conditions as claimed by many researchers? Moreover, are there any guidelines for umbilical cord blood banking? Let’s find out.
The use of autologous cord blood stem cells for therapeutic purposes has always been a cause of debate amongst many experts. Nonetheless, the guidelines put forth by ICMR confirms that the existing Umbilical Cord Blood Banking regulations are in par with those given in the Drugs & Cosmetic Act 1940 and Rules 1945 (Amendments 2016). It also further provides advice and guidance on the therapeutic use of umbilical cord-derived hematopoietic stem cells.1
This blog will help you understand all about the science behind storage of umbilical cord blood, the current status of its use in the healthcare sector, while also guiding you on various other issues associated with the subject. To begin with, let’s first unravel the definition of umbilical cord blood.
What Is Umbilical Cord Blood?
The umbilical cord blood refers to the blood (remnant) left behind in the placental blood vessels as well as in the umbilical cord (the tube-like structure that connects the baby to the mother during pregnancy inside the womb).
It is a rich source of stem cells and can be used to treat an array of hematopoietic and genetic disorders, including cancer.1
Therapeutic Use Of Umbilical Cord Blood


You must know that, unlike a bone marrow transplantation, umbilical cord blood (UCB) transplants are not very dependent on strict HLA matching criteria.
Therefore, an umbilical cord blood transplantation can come to your rescue here and be used as an alternative source of stem cells when children and young adults aren’t able to find other sources of HLA-compatible stem cell units.
It is to be noted that, ICMR recommends the use of umbilical cord blood for use in allogeneic hematopoietic cell transplantations.1 In fact, a hematopoietic stem cell transplantation using an individual’s own cord blood (autologous transplant), is not recommended for treatment of genetic disorders. Thus, the concept of private stem cell banking has become obsolete now.
Now, let’s have a quick look at some of the most common pros and cons associated with the use of umbilical cord blood as a source of stem cells.
Pros & Cons Of Umbilical Cord Blood As A Source Of Stem Cells
Pros:1
- Collection of cord blood is rather easy as compared to other stem cell sources
- It poses absolutely no risk to the mother or baby (during collection)
- The processing time of cord blood-derived stem cells is comparatively less
- It’s a lot more economical when compared to the collection of bone marrow stem cells
- The risk of transmitting infections to the recipient is also low
Cons:1
- Its use in children is limited owing to lower cell dose in the inoculum (final stem cell unit)
- Each units is of relatively smaller volume
- Unavailability of additional cell doses/units
- Storage difficulty at extremely low temperatures and unavoidable costs
While the cons associated with the use of cord blood as a source of stem cells cannot be ignored, the storage and processing conditions followed at LifeCell have already filled in these gaps. Furthermore, our Community Stem Cell Banking comprises an inventory of 75,000+ stem cell units and offers the highest chances (>97%) worldwide of finding acceptable matches for patients of Indian origin!2
Regulations For Use In India
The Central Drugs Standard Control Organisation (CDSCO) monitors the umbilical cord blood banks in India, while also providing licenses for them to operate. It is a constituent of the Ministry of Health and Family Welfare
There are 12 DCGI-certified umbilical cord blood banks in India at present, and among them, only 4 are AABB accredited - one of them being LifeCell’s Community Stem Cell Bank. Our bank is further accredited several times with agencies such as WHO, FDA, NABL, ISO, and many more.1,2
Moving forth, let’s now understand the common ICMR guidelines for umbilical cord blood banking when it comes to the collection, processing, and release of umbilical cord blood-derived stem cells.
Guidelines For Umbilical Cord Blood Collection, Transport, Processing & Release


1. Requirement for UCB Collection & Transport to UCB Bank1
- Adequate space for collection and temporary storage of UCB samples should be available.
- During the collection/handling of samples, universal precautionary steps should be followed to prevent contamination.
- A kit that comprises all the materials required for sample collection with clear instructions should also be available.
- The transportation of the kit should be done under prescribed temperatures.
- The documents collected from the client should record all details on the reagents used.
- The process of UCB collection should be carried out by the RMP / registered nurse / health care or medical professionals trained in the collection procedures
- The collected UCB should be aseptically placed in a disposable Polyvinyl chloride (PVC) bag that is procured from licensed manufacturers.
- The PVC bag should contain an adequate quantity of sterile pyrogen free anticoagulants like CPD, CPD-A (Citrate-phosphate dextrose/Citrate-phosphate-dextrose-adenine) and should be sealed well.
- The collection of UCB may either be in utero (after delivery of the infant but before delivery of placenta) or ex utero (after the placenta has been delivered).
- In order to protect cell viability and prevent damage of the UCB unit, temperature should be maintained.
- Shipping of UCB units should be done with utmost care, such that it withstands shocks and pressure changes during transportation, while also preventing sample leakage.
2. Requirement of Sample Processing, Cryopreservation & Storage1
- The umbilical cord blood units should be processed within 72 hours in a closed system, under clean conditions, and specified air quality with proper labeling.
- While being cryopreserved, the UCB units should be stored in appropriate freezing bags at temperatures less than or equal to -150°C.
- In order to maintain the stored UCB unit’s potency, stability, and viability, proper SOPs should be followed with minimal contamination risk.
- The stored units should be supervised closely and the temperature under which it is stored must be monitored every four hours.
3. Requirement for Release and Transport for Banked UCB1
- The different parameters of the processed UCB unit, including its potency, safety, integrity, and viability must be ascertained and documented before its release.
- When the UCB unit is being transported, the cold chain should continue to be maintained.
- The processed unit must also contain the proper shipping records that include the details of the unit like time of dispatch, intended recipient, as well as its destination.
- The safety of the paramedic who is involved in this process must also be considered.
It is worth noting that, at LifeCell, there are trained paramedics who assist in the collection of UCB units at birth, while also documenting the medical history of the donor infant (post birth) through a TRF. Our SOPs also include stringent quality checks of all chemical reagents that come in contact with the sample. Furthermore, the kits used are also highly protected as they’re transported in double walled stainless steel boxes.
From using the most advanced processing technology (Pentastarch), to taking utmost care when it comes to storage and transport of the banked stem cell units; the processing and release operations carried out by LifeCell aligns with the guidelines for umbilical cord blood banking laid out by the ICMR.2
It is also worth noting that, under LifeCell’s Safe Transplantation Program, every stem cell unit undergoes a free genetic screening test for 1500 inherited disorders before it is released for transplant.
The other benefits you can avail by enrolling for Community Stem Cell Banking with LifeCell include:
- More than 97% chance of finding a stem cell match within our registry
- Access to a stem cell inventory that comprises 75,000+ stored units
- Release of sample at no additional cost
- Safe and secure sample delivery under cryogenic conditions within cryo shippers
- Extended service to baby’s parents, siblings, and grandparents, and a lot more!
A Closing Note
As parents-to-be who are looking out for the best interest of their baby, it is important that you know the A to Z of any new service or test that you’re considering for your unborn child.
This blog is an attempt to educate you about the collection, processing, and release steps that comprises LifeCell’s Stem Cell Banking program and how it aligns with the guidelines for umbilical cord blood banking laid out by ICMR.
We hope you’re now well aware about the safety guidelines followed by LifeCell when it comes to banking your baby’s cord blood at birth. For any further queries, feel free to call us at 1800 66 55 33.
Are You/Anyone In Your Family Expecting A Baby?
If “Yes,” Please Share Your Expected Due Date